- December 19, 2021
- Posted by: Ganesh Shankar
- Category: Analytics
Applicable to Life Sciences / Manufacturing /Pharmaceuticals
In this blog, I wish to touch upon how analytics can play a vital role in companies that are bound to be compliant always as per change in the compliance and regulatory landscape.
By combining the value addition of external and internal data available in a company, they can enhance decision support system by being more driven driven. This decision making process ,I believe will also be shortened by using cleansed data available for business users’ consumption.
One such example is shown below is the FDA inspection dashboard built upon the publicly data available where we can see the trend of inspection per country and the type of outcome after an inspection.
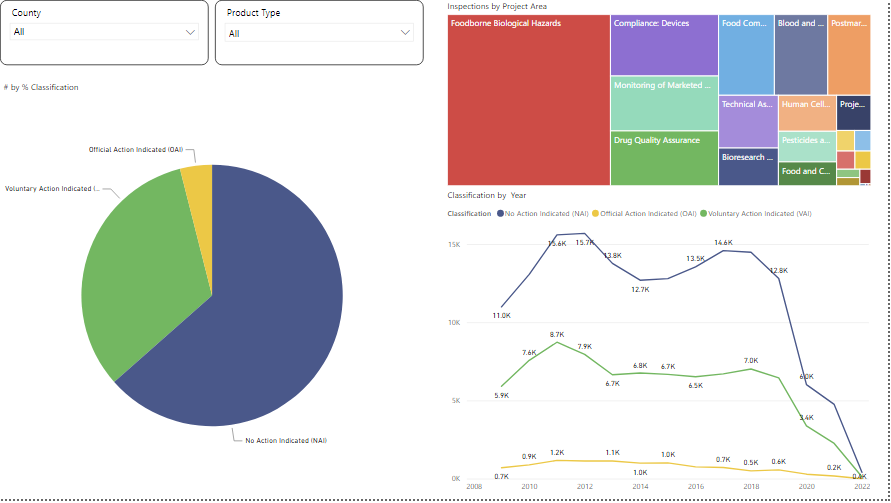
Data Courtesy: FDA Open Source
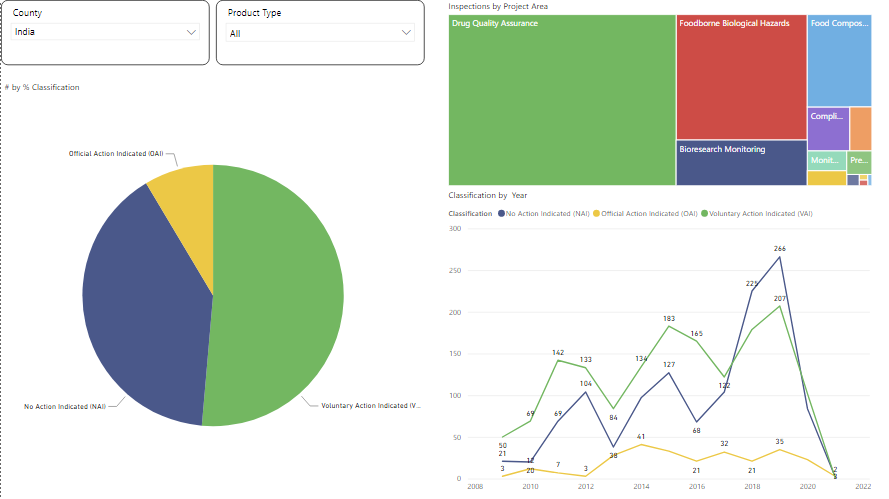
If you take country like India which is among top 5 exporter of generic medicine to the rest of world, We can see that the number of inspections has increased and the number of actions initiated has reduced, Which confirms the increasing compliance level as per international regulations, however a firm can use this data to internalize and validate their quality control, assurance and manufacturing standard operating procedures to suit the kind of actions/warning in a proactive approach to further reduce actions if any.
Trend shown below for India from FDA data during the period 2008 to 2021, where 8.57% of inspections have resulted in official actions initiated against the firm.
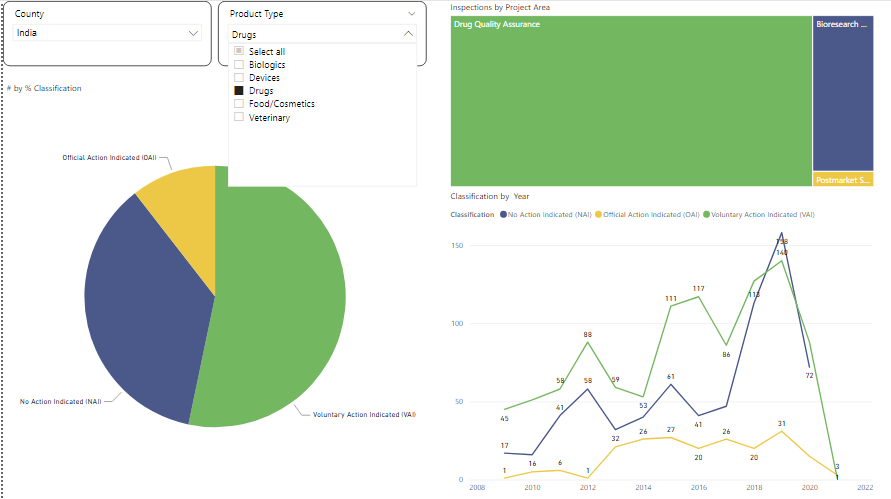
Also, if we analyse this open data further we can bring insight about the top areas where a firm can concentrate to mitigate any risk or inspection actions.
For example: for drugs more inspections are on the drug quality assurance and post-market surveillance
We , Cittabase provide analytics as a solution and deep dive into external factors which may lead to increase in use of data for their compliance and build data models which are curated to customer needs.


